Mainly practical applications: Plastic coatings on furniture, FRP composites (polyester, vinylester, epoxy, etc), gelcoat blisters in polyester laminates in boats, etc., though I do have 2 semesters of college chem (analytic for techs, inorganic for engrs). Yeah, I've used desiccants like P2O5 when using analytical balances...at least I think that's what it was. And I've been around HCl a few times -- my dad worked in a plant that used a train-car load of HCl (it came as a compressed gas, I believe) every day.
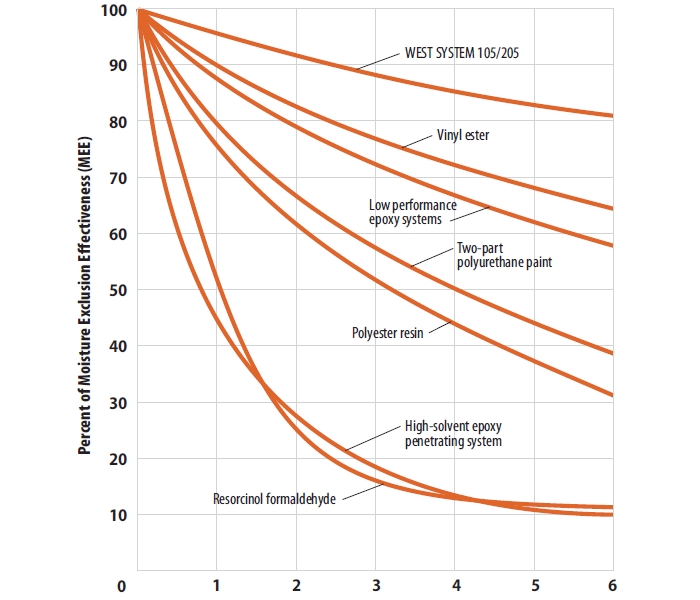
Yes, wax is very good, but it isn't perfect. USFS found that although the MEE for paraffin was higher than anything they tested, it was still only 70% when in air @ 80°F and 90% RH.
https://www.fpl.fs.fed.us/documnts/fplrp/fplrp462.pdf
Water generally goes where it wants to go. It's kinda like trying to stop the tides. Best thing to do is just get out of the way.